Clostridium butyricum CBM588®
Clostridium butyricum CBM588®: a butyrate resource directly from the colon
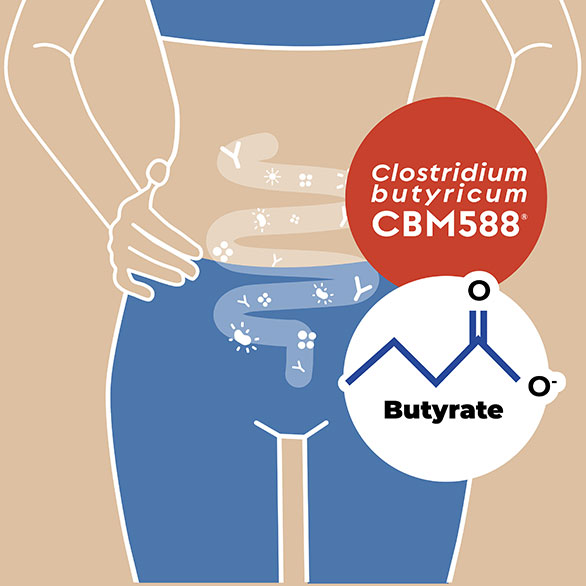
Clostridum butyricum is a bacterial species that owes its name to its great ability to produce butyrate.
Butyrate is a short-chain fatty acid that constitutes about 75 per cent of the energy source of colonocytes, the epithelial cells of the large intestine or colon. This substance is so important that a deficiency in it can even lead to intestinal distress and inflammation.
- butyricum is naturally present in about 20% of the intestinal microbiota of adults and can also colonise the intestines of infants. However, it is a bacterium that is very difficult to cultivate in vitro and, for this reason, scarcely used in the production of supplements or drugs.
A happy exception is the special strain called Clostridium butyricum CBM588®, isolated in Japan as far back as 1933, which has decades of successful use as a medicine in Japan and other Asian countries behind it.
The main characteristic of C. butyricum CBM588® is that it has been shown in several clinical studies to have the ability to produce large amounts of butyrate directly in the colon, through special metabolic pathways present in very few bacteria.
Another great ability of CBM588® is the promotion of an increased presence of other butyrate-producing bacteria in the gut, along with bifidobacteria and lactobacilli, which in synergy rebalance the entire bacterial ecosystem.
Butyrate produced by Clostridium butyricum was found to:
- Have Intestinal anti-inflammatory activity because it properly nourishes the colonocyte and thus enables the health of the intestinal epithelium
- Strengthen intestinal junctions and stimulates the production of intestinal mucus
- Allow proper reabsorption of sodium and water in the gut
In addition, Clostridium butyricum proved to be effective against diarrhea, in different clinical trials involving hundreds of patients with Irritable Bowel Syndrome with diarrheal bowel movements or patients with antibiotic-associated diarrhea. Moreover, it was also studied in patients with intestinal inflammation.
Thanks to its absolute safety studies, the use of C. butyricum CBM588® has been permitted in Europe since 2014, when it was granted Novel Food status by the European body. It is currently marketed as a food supplement in Italy.